Exercise and fitness
Exercise has short-term and long-term effects on the respiratory and cardiovascular systems, and on skeletal muscle.
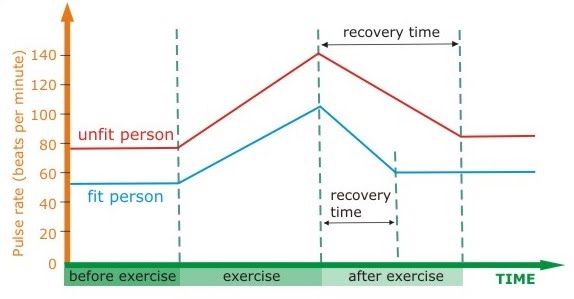
Short-term, heart rate as well as cardiac output, respiratory rate and tidal volume increase in order to provide more oxygenated blood to working muscles. These refer to how fast the heart beats, how much blood the heart pumps, how many breaths are taken and how much air is inhaled by the lungs.
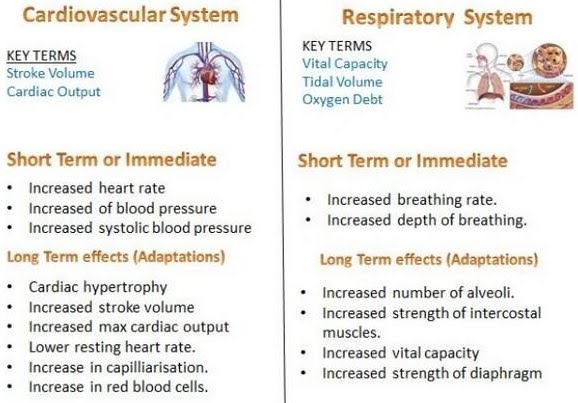
Blood vessels dilate in the muscles during exercise, while they contract in the digestive system. This blood shunting prioritises the most active body systems.
Longer term effects such as cardiac hypertrophy and skeletal muscle hypertrophy (growth) from certain kinds of exercise result in increased fitness, and an overall improved health. Regular moderate or vigorous exercise is associated with a lower incidence of many diseases including heart disease, stroke, type 2 diabetes, colon cancer, breast cancer, early death, osteoarthritis, hip fracture, depression and dementia.
Aerobic fitness, that is the efficiency with which the oxygen-dependent processes take place during exercise e.g. heart, lung and muscle function, is associated with multiple factors such as participation in exercise (aerobic exercise increases aerobic fitness), sex and age.
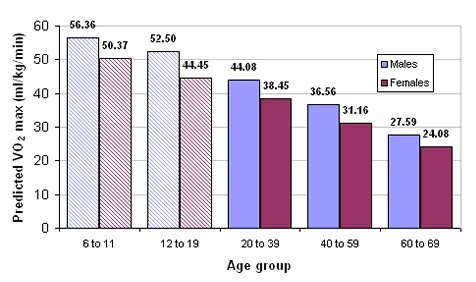
Aerobic fitness, as measured by the VO2 max (maximum uptake of oxygen) falters with age, and averages out higher in males compared to females.
Maximum oxygen uptake does what it says on the tin and is denoted by VO2 max which refers to the maximum volume of oxygen.
It represents the amount of oxygen used up through breathing during incremental exercise that exerts the body to that maximal point. For example, a patient or athlete can be hooked up to a respirometer that records ventilation (breathing), oxygen concentration and carbon dioxide concentration in the air inhaled and exhaled, while using a treadmill.

The data can be expressed as volume of oxygen per time i.e. litres of oxygen per minute, L/min, or more adjusted for weight in volume of oxygen per weight per time i.e. litres of oxygen per kilogram per minute.
For example, the highest recorded VO2 max was 97.5 mL/(kg*min) in cyclist Oskar Svendsen.
Maximum oxygen uptake is a measure of cardiovascular and aerobic function used in the world of sport as well as in healthcare. For some patients who would benefit from not undertaking the interval exercise method of measuring VO2 max, alternative methods are used. These do not get to measure the actual maximum itself, and are therefore sum-maximal tests.
Training is another key factor that determines aerobic fitness, and it has the power to significantly improve it. The F.I.T.T. factors of the exercise programme regulate the fitness outcome. These are frequency, intensity, type and time.
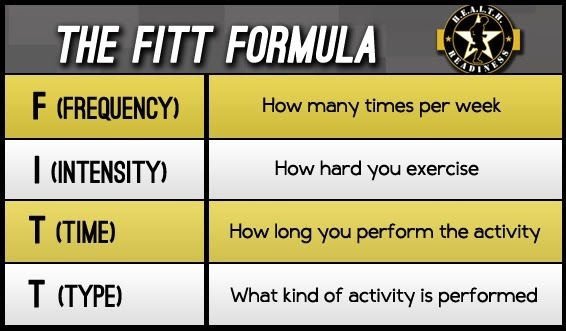
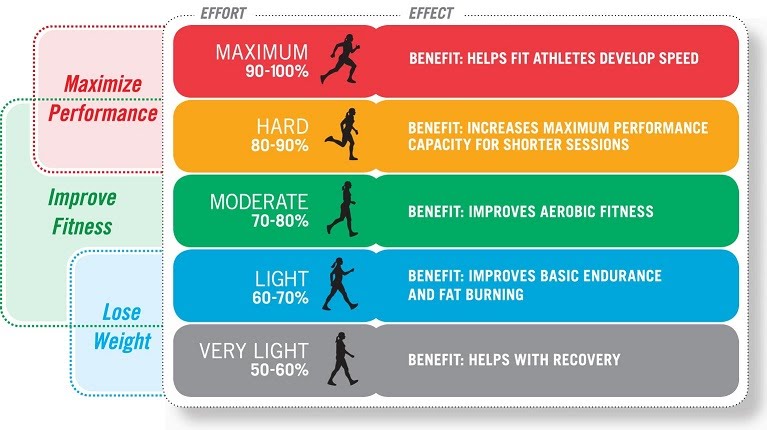
The type of activity could be running, cycling, team sports, etc. while the frequency, intensity and time spent doing it increase as the aerobic fitness improves. A guideline starting programme might be 30 minutes of moderate intensity exercise 3 times a week. More specific measures of intensity for aerobic exercise include the percentage of target heart rate to be exercised. For example, exercising 120 beats per minute out of a target of 140 beats per minute would be equivalent to a 86% intensity.
Keeping track of the progress of exercise and aerobic fitness can be done by monitoring resting heart rate (the higher the aerobic fitness, the lower the resting heart rate), breathing rate and recovery time.
Supplementation for the purpose of enhancing athlete performance can be undertaken in many ways. One of them is blood doping which involves increasing the amount of red blood cells available in the blood, to improve oxygen availability and delivery to cells. One method is taking an athlete’s own blood and storing it for later transfusion. Another method is taking recombinant human eryhtropoietin (rhEPO) which is a hormone that stimulates additional red blood cells production in the body.
Blood doping has been banned in order to allow for fair competitions in endurance sports.
Some steroids are also banned. Steroids are a class of small chemicals that play key roles in metabolism and health e.g. the sex hormones testosterone and estrogen. Some steroids (anabolic-androgenic steroids, AAS), testosterone included, are banned or regulated because they can have effects pertaining to athletic performance. Analogues and chemicals not normally present in the human body (trenbolone) can also be used for this purpose. These varying chemicals have different effects, and some are more dangerous than others. The response to steroids can be very different between individuals.
Another supplementation strategy is carbohydrate loading. This is done by very high endurance athletes such as those running or playing sports for more than 60-90 minutes. Carb loading involves eating a large amount of carbohydrate-rich foods in order to maintain and increase the glycogen stores of the body i.e. muscles, liver. Before the endurance event, athletes decrease the amount of training undertaken to prevent glycogen depletion, and shortly (12-18 hours) before the event they load with carbohydrates.
Haemoglobin
Carbon dioxide and oxygen are transported in the blood under different conditions. Carbon dioxide is more soluble than oxygen, so some of it is directly present in the blood plasma. It can also be present as bicarbonate ions which increase blood acidity and signal if there is too much CO2 in the blood and not enough O2.
Finally, both CO2 and O2 can bind to haemoglobin which is present in red blood cells. Its function is the oxygenation of tissues, as oxygen is central to aerobic respiration, the metabolic process of creating chemical energy for all cell functions. Its smaller cousin, myoglobin, stores rather than transports oxygen in the body, and instead of being found in the blood, resides in muscle tissue. It only has one haem group so can only bind one oxygen molecule, while haemoglobin binds up to 4.
Haemoglobin is a type of protein. It is present in many varied organisms on our planet, and has a similar chemical structure in all of these organisms. In humans, haemoglobin is found in red blood cells. Haemoglobin’s function is the transport of oxygen around the body. Oxygen must reach all parts of our bodies because it is required in the process of cellular respiration (to produce ATP – the main molecule involved in releasing energy for all uses).
Haemoglobin is a big deal, so naturally, it has a quaternary structure (multiple protein chains linked together to form a greater functional unit which also includes inorganic molecules). Just stare in awe at this beauty:
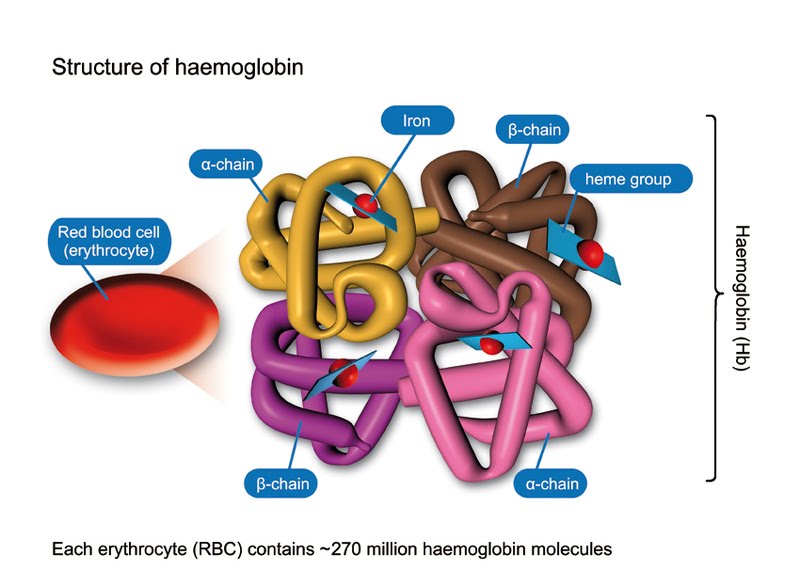
Erythrocyte is a very easy to remember name for a red blood cell. As you can see, there are 4 protein chains: 2 alpha chains and 2 beta chains. At the centre of each chain there is a haem (or heme) group which is an inorganic group containing one iron ion. The million pound question, of course, is how many iron ions are there in one haemoglobin molecule? (No, you will not be lucky enough to get that question for 10 marks in any exam.)
While haemoglobin has 4 distinct chains, myoglobin is only made of one chain, hence can carry 1 oxygen molecule rather than 4.
If you think this is all nice and easy, read on to be utterly disappointed.
Despite the fact that haemoglobins are similar in structure and function across a variety of organisms, they are adapted to different needs, as organisms span the large breadth of the biosphere. Some organisms need to be able to make use of very little oxygen available in their environment.
Since there are 4 iron ions in each haemoglobin molecule, and iron ions are directly responsible for binding the oxygen molecules (hence a lack of iron may cause anaemia), a haemoglobin molecule which has 4 oxygens bound to its haem groups is called saturated. The % saturation of haemoglobin overall in an organism is used to determine haemoglogin’s ability to bind oxygen. This ability is affected by the partial pressure of oxygen (partial because it is only 21% of air).
This is a graph showing the oxygen dissociation curve:
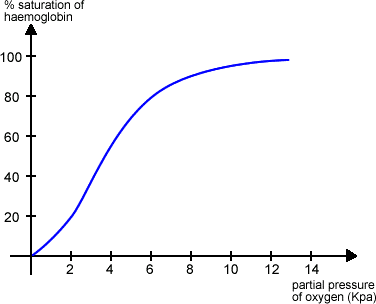
As you can see, the higher the partial pressure of oxygen (which reflects the amount of oxygen), the higher the % saturation of haemoglobin. Haemoglobin binds oxygen in the lungs where the partial pressure of oxygen is high. Of course, more oxygen than could be taken up by haemoglobin is irrelevant because the limiting factor becomes the number of haemoglobin molecules (hence the curve plateaus at about 8 KPa).
A very important point to take from the graph is that at a low partial pressure of oxygen (for example in respiring tissues), the % saturation of haemoglobin is decreasing. What this means is that haemoglobin has the unique ability to release oxygen where it is needed most. Abundance of oxygen in the lungs? Haemoglobin FETCHES! Tissues depleted of oxygen? Haemoglobin SPITS! Of course you will use terms such as oxygen binding and releasing, and percentage saturation of haemoglobin in your exams, won’t you.
Let’s go back to one of the first points on this topic. I said that haemoglobin action varies slightly between different organisms, depending on their individual needs and environment. This is a crucial point because those evil examiners will put a scary looking double, or even triple curve graph in front of you and ask you to explain what is going on. Looking at the above graph, you can see that at 6 KPa the % saturation is 80. This may suit an organism fine, but others may well have shifted curves, where the points at which haemoglobin binds and releases oxygen are different. These curves may well be shifted to either the left or the right. This is what the graph might look like:
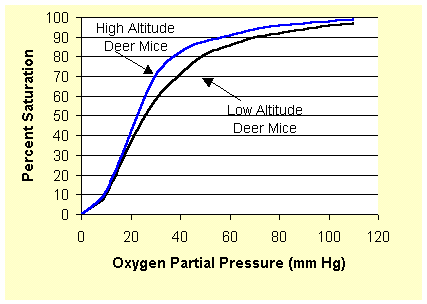
If we take, say, 80% saturation, the corresponding partial pressure of oxygen is about 35 mm Hg for high altitude deer mice, and about 50 mm Hg for low altitude deer mice. What does this mean? It means that high altitude deer mice’s haemoglobin binds oxygen at a lower partial pressure than that of low altitude deer mice. Essentially, their haemoglobin is able to make better use of less oxygen.
Why?
Well, let’s think, what do we know of these mice? Some live at high altitudes, and others live at low altitudes. What do we know about altitudes that is relevant? We know that there is less oxygen at higher altitudes in the atmosphere. Hence the haemoglobin of high altitude deer mice has evolved to bind oxygen at lower partial pressures – where there is less of it available. This confers a clear advantage in terms of survival.
Myoglobin has an even higher affinity for oxygen, as its role is storing it in muscle.
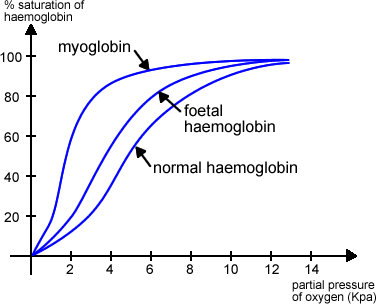
Foetal haemoglobin has a slightly higher affinity compared to adult haemoglobin. It is a different structure with two alpha chains and two gamma chains as opposed to alpha plus beta. It’s used by foetuses and babies up to 6 months after birth, when the gradual switchover to adult haemoglobin takes place.
During gestation, the foetus relies on external delivery of oxygen and removal of carbon dioxide across the placenta. The only way of making sure this happens preferentially towards/away from the foetus is to have haemoglobin with a higher affinity for oxygen, so that it is taken up as it becomes present near the placenta. This wouldn’t happen if the affinities were equal.
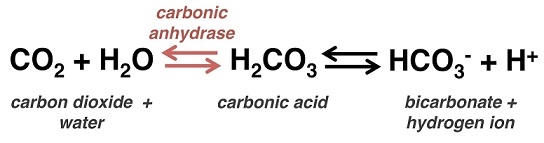
Right, onto the last bit now (phew). You will be expected to know the effect of carbon dioxide (CO2) on the oxygen dissociation curve. CO2 being acidic decreases blood pH if in increasing quantity, and it results in carbamino compounds being produced. These compounds bind to haemoglobin and shift the curve to the right. Bicarbonate ions (formed from the reversible reaction catalysed by carbonic anhydrases) also contribute to a shift to the right, as they release protons into the blood plasma. This is known as the Bohr effect.
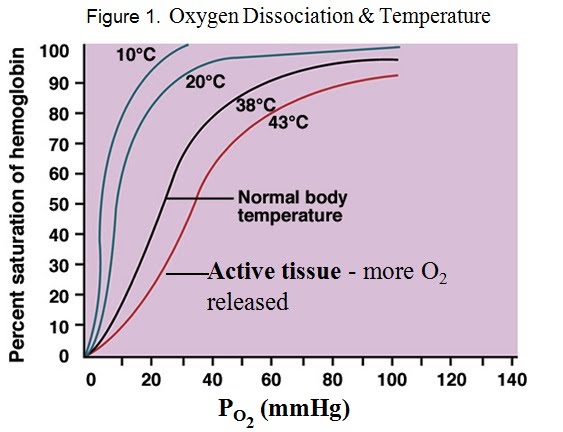
Alongside carbon dioxide and pH, temperature affects the oxygen dissociation from haemoglobin. Higher temperature shifts the dissociation curve to the right. This means more oxygen dissociates quicker at a higher temperature. For example, the 50% saturation point for body temperature is around 20 mm Hg (partial pressure of oxygen), while that of warmer, active tissue is around 30 mm Hg.
The terms oxygen deficit and excess post-exercise oxygen consumption (EPOC, formerly “oxygen debt”) are used to describe the differences between required oxygen during exercise and required oxygen following exercise in order to restore the body to its resting state and function.
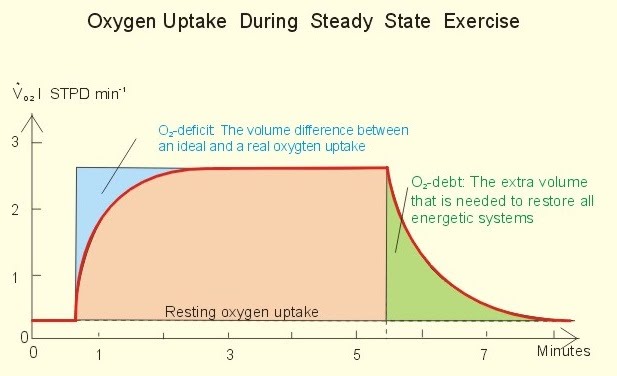
EPOC is greater than the oxygen deficit, hence it is no longer accurate to describe it as “debt”. The reason it is not simply making up for the exercise done anaerobically during a lack of sufficient oxygen intake, is that further energy expenditure is put towards metabolic processes such as higher tissue activity due to overheating from exercise, and sustained intensity of heart and respiratory muscles that are working harder than the rest.
Skeletal muscle
How do muscles contract? Glad you should ask. Look in any book and you’ll probably be scarred and put off completely. I know I was! I mean what is the deal with Z line, A band, I band…?!
So let’s look into it very gently indeed. First off, a good idea about what muscles look like on a bigger scale:
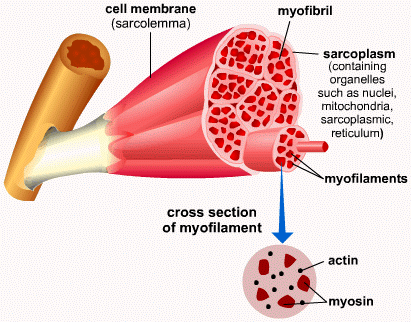
The muscle is attached to the bone (spot the nice bone marrow) by the tendon. Muscle cells have a plasma membrane and cytoplasm much like any other cells, but each has a special name. The membrane is called sarcolemma while the cytoplasm is called sarcoplasm (it has a much higher glycogen storage content).
The basic unit of muscle tissue is the myofibril which has a tube-like shape and is made up of the muscle cells themselves. These look fibrous and are organised into myoflilaments.
The bread and butter of muscle cells (more bread -11% protein- and less butter!-1% protein) are 2 types of protein that work together to cause contraction. Because muscle contracts, never “extends”. That’s why our arms and legs have muscles on opposing sides, so that that contraction of each may result in either a “push” motion or a “pull” motion.
On a smaller scale, myofibrils contract as a result of those 2 proteins sliding along one another. Those 2 proteins damn right deserve a name, so let’s call them actin and myosin just because that’s what everyone else who speaks English and has any awareness whatsoever of them calls them. Actin appears lighter under a microscope than myosin, so a lovely colour pattern can be observed.
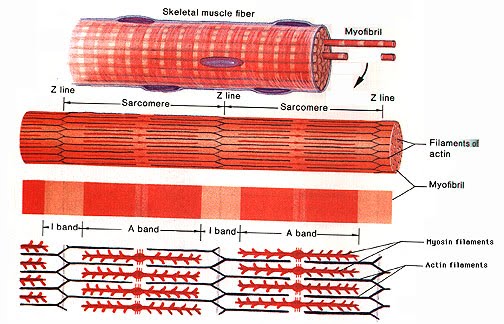
The lovely colour pattern is bordered by Z discs or lines, named so due to some German word for “between”. Seriously… That’s why they’re called Z discs, and since I am beyond disappointed with that, I shall conclude that the lines themselves being in a zig-zag in the above diagram, the Z line mystery has found a better explanation.
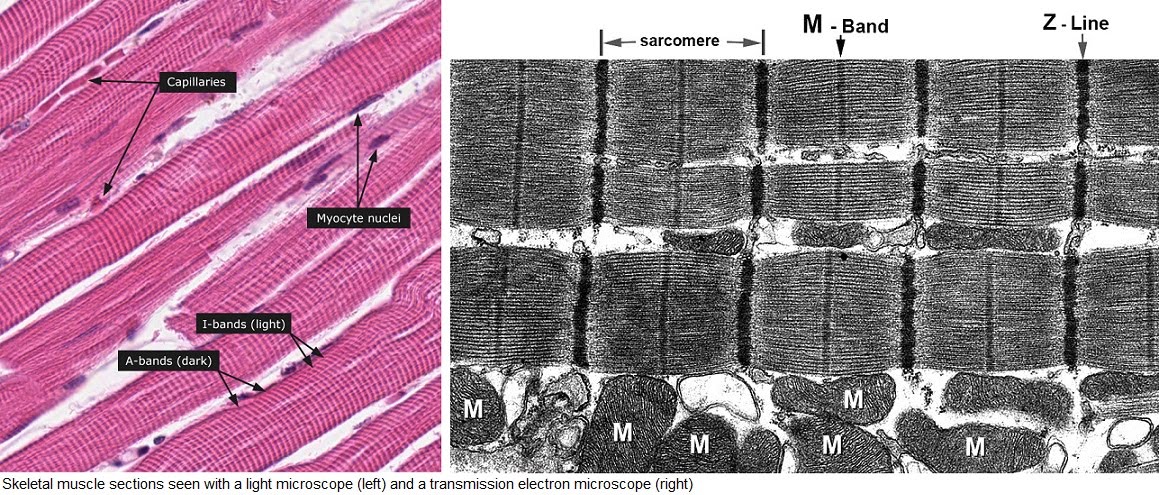
The segment in question is called a sarcomere. All these “sarco-” terms for muscle parts (sarcolemma, sarcoplasm, sarcomere) come from the Greek “sarx” which means flesh. Now see, that makes a bit more sense.
During contraction, the myosin doesn’t actually get shorter. It is the actin that slides over to decrease the overall length of the sarcomere.
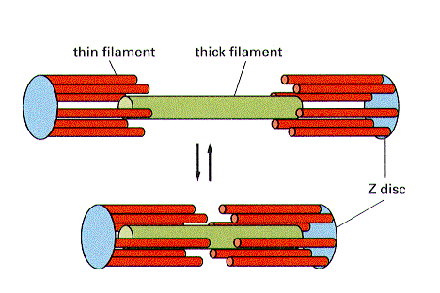
This is a really good diagram for understanding the basic principle of actin (thin filaments) sliding over the myosin (thick filaments) to shorten the sarcomere and achieve contraction. If you understand this, you won’t be shocked by the inevitable trippy questions that examiners will hurl at you in the exam about fancy H/I/A bands/lines/zones. These randomly named lines/zones/bits/pieces are totally arbitrary. If you must know, the A band and I band referring to the length covered by myosin and actin respectively were established as a result of how they appear under a microscope.
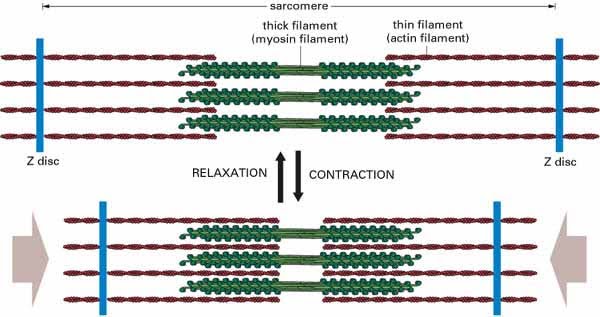
Another diagram for the visually-inclined.
Let’s go back to this for a second:

What happens to the I band during contraction? It gets shorter. What about the A band? It stays the same.
Notice the wiggly heads on the myosin above. They look like branches, but they’re wiggly heads trust me. The wiggly heads are actually what enables the myosin to pull along the actin during contraction. Bridges are formed following the ATP breakdown reaction:
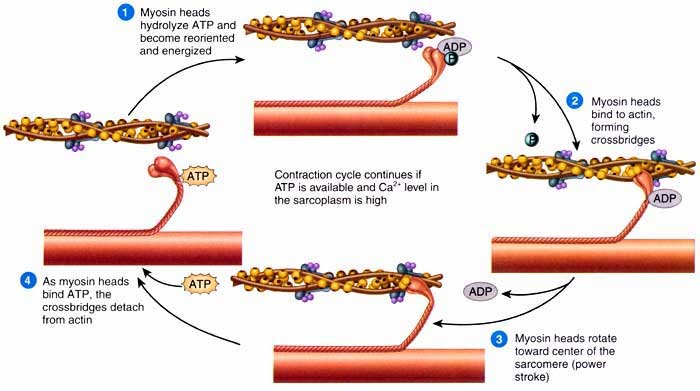
The crossbridges formed are called actinomyiosin bridges. It’s important that when muscles are relaxed no power strokes occur by the myosin. Otherwise they wouldn’t be that relaxed, would they?
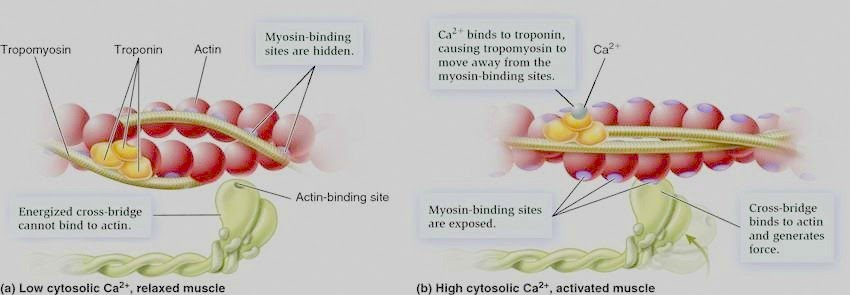
Drum roll please for our latest protein addition: tropomyosin. “Tropo” means turn, so I guess you can think of tropomyosin as a turn of myosin in the sense that it either enables it to bind actin or it turns it away… Good one? Shall grab my coat…
Calcium ions are very important in this process as it is their initial binding (to troponin) that results in tropomyosin moving so that the binding sites on actin are exposed.
Experiments can be carried out to test muscle contraction. A few isolated muscle fibres (or even single fibres) are placed under a microscope, and varying combinations of ATP and metal salts such as KCl and MgCl2 are added. These are essential for contraction, so the hypothesis is that the fibres will contract when all are added, but not when they are added alone.
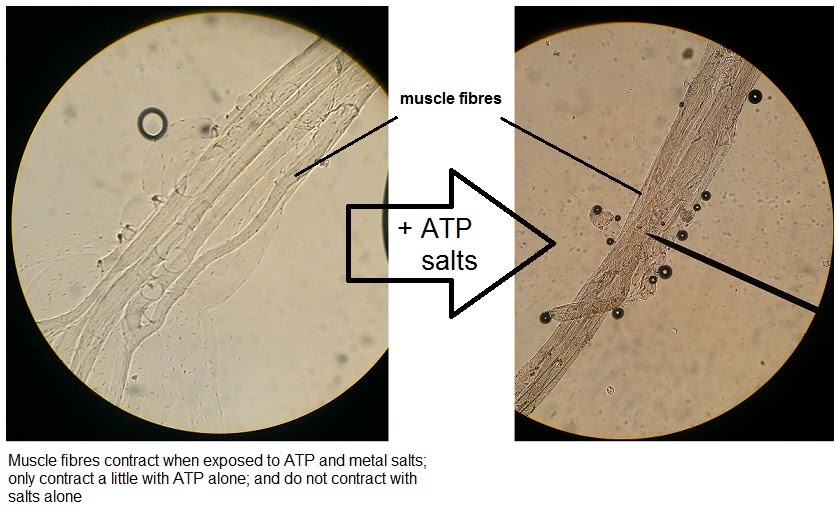
The highest degree of contraction is observed when ATP and the salts are added together, especially in few or single fibres where contraction is more noticeable compared to bigger samples of muscle. ATP alone still yields a small degree of contraction, however salts alone do not. This is because ATP is essential to contraction.
Calcium ions are not required in the experiment because the muscle samples contain glycerol (for preservation prior to experimentation) which interferes with troponin and tropomyosin, allowing for contraction. However, the metal salts increase contraction with ATP significantly compared to ATP alone by enabling stronger bonds between actin and myosin.
Ok byeeeeee
